International nomenclature of alkanes. Alkanes: structure, properties
It is Useful to start with the definition of alkanes. It's saturated, or saturated hydrocarbons, paraffins. You can also say that this carbon, in which the connection of the C atoms is carried out by means of simple relations. The General formula is: CnH₂n+ 2.
It is Known that the ratio of C and H atoms in their molecules as if to compare with other classes. Due to the fact that all valence occupied either C or H, chemical properties of alkanes expressed insufficiently bright, so their second name is the phrase the ultimate or saturated hydrocarbons.
There are Also more ancient name which best reflects their relative heinerfest-paraffins, which means “devoid of affinity”.
So, the topic of our conversation today: “Alkanes: homologous series, nomenclature, structure, isomerism”. Will also be presented data about their physical properties.
Alkanes: structure, nomenclature
They C atoms are in such a condition as sp3 hybridization. In this regard, a molecule alkanes can be demonstrated as a set of tetrahedral structures C, which are connected not only among themselves but also with H.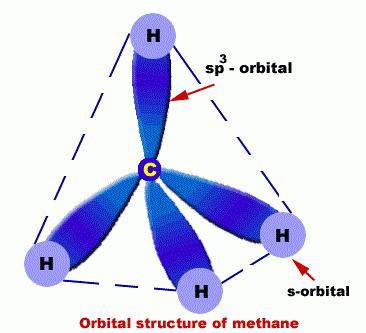
Between the atoms C and H are durable, very low-polarity and a s-bond. The atoms around simple relationships always revolve, as a consequence, molecules of alkanes are varied, and the bond length, angle between them constant values. Forms that transformirovalsya each other due to the rotation of the molecule around σ-bonds, so called conformations.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
In the process of separation of H-atom of the molecule form a 1-valent particles called hydrocarbon radicals. They are the result of connections not only organic substances but also inorganic. If you take away 2 hydrogen atoms from the hydrocarbon molecules of the limit, we get 2-valent radicals.
Thus, the nomenclature of alkanes can be:
- Radial (old version);
- Substitution (international, systematic). Proposed IUPAC.
Features radial item
In the first case, the nomenclature of alkanes is characterized by the following:
- Consideration of hydrocarbons as derivatives of methane, which is substituted by 1 or more H atoms of the radicals.
- High degree of convenience in the case is not very complex compounds.
Features of substitutive nomenclature
Substitutive nomenclature of alkanes has the following features:
- The Basis for the name – 1 carbon chain, the remaining molecular fragments are treated as deputies.
- If there are multiple identical radicals in front of their name indicates the number (in words), and radical numbers are separated by commas.
Chemistry: nomenclature of alkanes
For convenience, the information is presented in a table.
Compound Name | Basis of the name (root) | Molecular formula | Name carbon Vice | Formula carbon Vice |
Methane | Met- | CH₄ | Methyl | CH₃ |
Ethan | Et | C₂H₆ | Ethyl | C₂H₅ |
Propane | Prop | C₃H₈ | Propyl | C₃H₇ |
Bhutan | But- | C₄H₁₀ | Butyl | C₄H₉ |
Pentane | Penta- | C₅H₁₂ | Pentyl | C₅H₁₁ |
Hexane | Hex- | C₆H₁₄ | Exil | C₆H₁₃ |
Heptane | Gept | C₇H₁₆ | Heptyl | C₇H₁₅ |
Octane | Oct- | C₈H₁₈ | Octyl | C₈H₁₇ |
Noonan | Non- | C₉H₂₀ | Neil | C₉H₁₉ |
Dean | Dec- | C₁₀H₂₂ | Decyl | C₁₀H₂₁ |
The Above nomenclature of alkane involves the names which have developed historically (the first 4 members of a number of saturated hydrocarbons).
The names of nondeployed alkanes with 5 or more C atoms is formed from the Greek numerals which represent the number of atoms C. thus, the suffix -EN says that the substance of the number of saturated compounds.
In compiling the names of deployed alkanes in the role of the main chain is selected which contains maximum number of atoms C. It is numbered so that the substituents was the smallestnumber. In the case of two or more chains of equal length the main getting the one that contains the largest number of deputies.
Isomerism of alkanes
As the hydrocarbon, the ancestor of the row is the methane CH₄. With each subsequent representative of the methane number of observed contrast to the previous methylene group - CH₂. This pattern can be traced in the entire series of alkanes.
The German scholar Shil proposed to call this series homology. In Greek means "similar, similar”.
Thus, homologous series – a set of related organic compounds, having similar structure with similar himsostav. Homologues of members of this series. Homological difference – a methylene group, which are different for 2 adjacent homologue.
As mentioned earlier, the composition of any saturated hydrocarbon can be expressed by the General formula CnH₂n + 2. So, following the methane member of the homologous series is ethane - C₂H₆. To deduce the structure of methane must be replaced with 1 H atoms in CH₃ (figure below).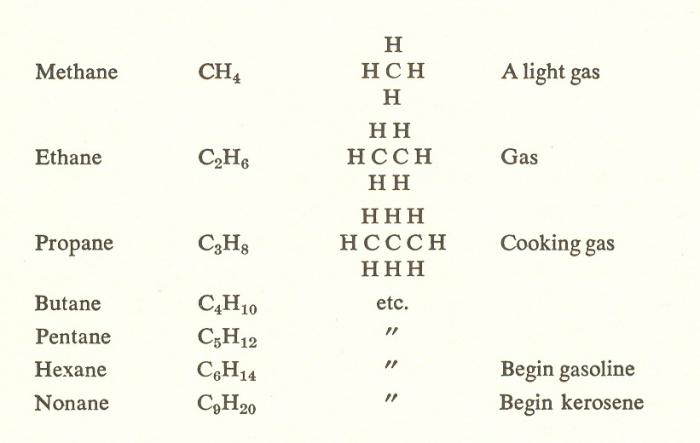
The Structure of each subsequent homologue can be derived from previous in the same way. As a result of the formed ethane propane C₃H₈.
What is isomers?
Are substances that have identical qualitative and quantitative molecular composition (identical molecular formula) but different chemical structure, as well as with different himsostav.
By the aforementioned hydrocarbons differ in the parameter boiling point: -0,5 ° C; and butane, -10° - isobutane. This type of isomerism is called as isomerism of the carbon skeleton, it refers to the structural type.
The Number of structural isomers increases rapidly with increasing number of carbon atoms. Thus, C₁₀H₂₂ will match 75 isomers (not including space), and for C₁₅H₃₂ already known 4347 isomers, for C₂₀H₄₂ - 366 319.
So it became clear that such alkanes, homologous series, isomerism, nomenclature. Now it's time to move on to the rules of making names by IUPAC.
Nomenclature IUPAC rules of formation of names
First, you need to find in the hydrocarbon structure of the carbon chain, which is the longest and contains the maximum number of substituents. Then you must number the C atoms in the chain, starting from the end, which is closest Deputy.
Second, the basis – the name of an unbranched saturated hydrocarbon of which the number of C atoms corresponds to the main circuit.
Third, before basis must indicate the number of lokantas, which are located near the Vice. Behind them are written with a hyphen the names of the deputies.
Fourth, in the case of identical substituents at different C atoms lokanta are combined, before the name appears multiplying prefix di-for two identical substituents, three-for three, Tetra-four Penta-five, etc. Numbers should be separated by a comma, and the words – with a hyphen.
If the same C atom contains two Deputy, locant, too, is written twice.
According to these rules and formed the international nomenclature of alkanes.
Newman Projections
This American scientist invited for a graphic demonstration of the conformations special projection formula projection Newman. They correspond to the forms A and B and is shown below.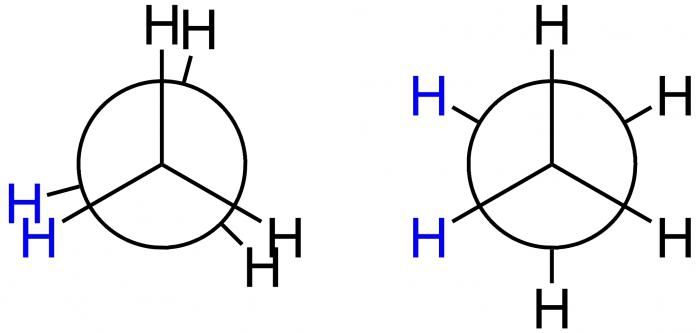
In the first case, And-eclipsed conformation, and the second B-inhibited. In position A, the H atoms are located at a minimum distance from each other. This form corresponds to the largest value of the energy, due to the fact that the repulsion between them is largest. It is energetically unfavorable condition, so that the molecule tends to leave it and move on to a more stable position B. Here the H atoms are most removed from each other. Thus, the energy difference of these provisions – 12 kJ/mol, allowing free rotation around the axis in a molecule of ethane, which connects the methyl group, it turns out uneven. After being hit in the energetically favorable position of the molecule there is delayed, in other words, “retarded”. That is why it is called inhibited. The result – 10 thousand molecules of ethane are in the form of an inhibited conformation under the condition of room temperature. Only one has another form - hidden.
Obtaining saturated hydrocarbons
From the article it became known that the alkanes (structure, nomenclature they are described in detail earlier). It will be useful to consider ways of obtaining them. They are distinguished from such natural sources as oil, natural and associated gas, coal. Also apply synthetic methods. For example, H₂ 2H₂:
- The Process of hydrogenation of unsaturated hydrocarbons: CnH₂n (alkenes)→ CnH₂n+2 (alkanes)← CnH₂n-2 (alkyne).
- Mixture of C and H monoxide - synthesis gas: nCO+(2n+1)H₂ → CnH₂n+2+nH₂O.
- From carboxylic acids (or their salts): electrolysis at the anode, at the cathode:
- Electrolysis Kolbe: 2RCOONa+2H₂O → R-R+2CO₂+H₂+2NaOH;
- Reaction Dumas (alloy with alkali):CH₃COONa+NaOH (t) → CH₄+Na₂CO₃.
- The Cracking of oil: CnH₂n+2 (450-700°)→ CmH₂m+2+ Cn-mH₂(n-m).
- Gasification of the fuel (solid): C+2H₂ → CH₄.
- Synthesis of complex alkanes (halogenated derivatives), which have fewer C atoms: 2CH₃Cl (harmatan) +2Na → CH₃ - CH₃ (ethane) +2NaCl.
- Decomposition of water petanidou (metal carbides): Al₄C₃+12H₂O → 4Al(OH₃)↓+3CH₄↑.
Physical properties of saturated hydrocarbons
For convenience the data are grouped in the table.
Formula | The Balkans | The melting point in °With | Boiling point in °C | Density, g/ml |
CH₄ | Methane | -183 | -162 | 0,415 at t = -165° |
C₂H₆ | Ethan | -183 | -88 | 0,561 at t= -100°C |
C₃H₈ | Propane | -188 | -42 | 0,583 at t = -45°C |
N-C₄H₁₀ | N-butane | -139 | -0,5 | 0,579 at t =0°C |
2-Methylpropane | - 160 | - 12 | 0,557 at t = -25°C | |
2,2-Dimethyl-propane | - 16 | 9,5 | 0,613 | |
N-C₅H₁₂ | N-Pentane | -130 | 36 | 0,626 |
2-Methylbutan | - 160 | 28 | 0,620 | |
N-C₆H₁₄ | N-Hexane | - 95 | 69 | 0,660 |
2-Methylpentan | - 153 | 62 | 0,683 | |
N-C₇H₁₆ | N-Heptane | - 91 | 98 | 0,683 |
N-C₈H₁₈ | N-Octane | - 57 | 126 | 0,702 |
2,2,3,3-Tetra-methylbutane | - 100 | 106 | 0,656 | |
2,2,4-Trimethyl-pentane | - 107 | 99 | 0,692 | |
N-C₉H₂₀ | N-Nonan | - 53 | 151 | 0,718 |
N-C₁₀H₂₂ | -Dean | - 30 | 174 | 0,730 |
N-C₁₁H₂₄ | Formica | - 26 | 196 | 0,740 |
N-C₁₂H₂₆ | N-Dodecane | - 10 | 216 | 0,748 |
N-C₁₃H₂₈ | N-Tridecan | - 5 | 235 | 0,756 |
N-C₁₄H₃₀ | N-Tetradecane | 6 | 254 | 0,762 |
N-C₁₅H₃₂ | N-Pentadecane | 10 | 271 | 0,768 |
N-C₁₆H₃₄ | N-Hexadecan | 18 | 287 | 0,776 |
N-C₂₀H₄₂ | N-Eicosan | 37 | 343 | 0,788 |
N-C₃₀H₆₂ | N-Triacontane | 66 | 235 and 1 mm Hg. St | 0,779 |
N-C₄₀H₈₂ | N-Tetracontyl | 81 | 260 and 3 mm Hg. article. | |
N-C₅₀H₁₀₂ | N-Pentaconta | 92 | 420 and 15 mm Hg. article. | |
N-C₆₀H₁₂₂ | N-Hexacontane | 99 | ||
N-C₇₀H₁₄₂ | N-Heptacosane | 105 | ||
N-C₁₀₀H₂₀₂ | N-Haktan | 115 |
Conclusion
The article was considered such a concept as alkanes (structure, nomenclature, isomerism, homologous series, etc.). Little is told about osobennostyah radial and substitution items. Described methods of obtaining alkanes.
In addition, the article lists all nomenclature of alkanes (the test can help to learn the information).
Article in other languages:
HI: https://www.tostpost.com/hi/education/12510-alkanes.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
Functions of the pharynx human
it is not Surprising that the throat is called the "main gate" into the human body, because everything that comes in, goes through the organ. It is commonly referred to simply as "throat", but in medical terminology it has a diffe...
the Society and its structure – one of the Central problems in sociology. Some textbooks even define it as a subject of science. Any society – is not a monolith, not something homogeneous. It consists of va...
Each of us are still at school is familiar with this part of speech as an adverb. We actively used them in daily speech, not thinking about any rules or fundamentals of the theory.However, you need to understand the concept: what ...
Veronica Franco, courtesan and poet: a biography
Veronica Franco – the most famous woman of the Renaissance. This famous courtesan famous for the fact that men have dreamed not so much about her body, but about the opportunity to talk with her, to gain her attention, to en...
Is it possible to influence heredity? Behaviour and genes
Perhaps, everyone ever heard this phrase: "like father like son", "Apple from the tree...", "my mom is like". All of this suggests that people are noting the similarities akin. Human heredity - the ability of an organism at the ge...
In 1938, at the literary magazine “New world” was published story Platonov "Olga". Later the author gave this work another name – “At the dawn misty youth”. This story – the story of a...





















Comments (0)
This article has no comment, be the first!