Formic aldehyde. Obtaining formic aldehyde
Formic aldehyde, or formaldehyde-is a colorless gas having a pungent, unpleasant smell. It is highly soluble in water and in alcohols. Formaldehyde is very toxic and can cause in the human body pathological changes. In addition, it is considered a carcinogen.
Formaldehyde – the first member of the whole homologous series, which includes aliphatic formic aldehyde. Formic acid also exhibits the properties of that group.
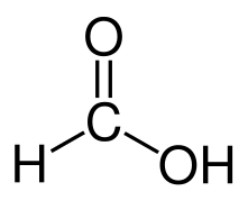
Chemical properties
Formaldehyde is able to join in all the reactions, which are characteristic of homologous series of aliphatic aldehydes. Including with nucleophiles. He also is associated with reducing reagents. This is mainly due to the fact that the formaldehyde of low electron density on the carbon atoms. Thanks to this structure it is very easy to react with even weak nucleophiles. This explains the fact that in aqueous solutions of formic aldehyde is detected in gidratirovannom condition.
Industrial Production

This substance is a simple formula. Formic aldehyde for chemical language is: HCHO. The main industrial method of its preparation is the oxidation of methanol. This reaction is carried out using a silver catalyst. The required temperature is 650 degrees. Oxidation of methanol occurs at atmospheric pressure.
This process for quite a long time and are used everywhere. It is well mastered. Approximately 80% of formaldehyde is obtained through the oxidation reaction of methanol. However, this is not the only way. Recently was developed a more promising method. It is based on the use of an iron-molybdenum catalysts. This decreases the level of required temperature up to 300 degrees, which is almost twice less.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
Also known another industrial method – the oxidation of methane. The reaction to this fairly simple formula: formic aldehyde is obtained under a pressure of 1-2 MPa at a temperature of 450 degrees. As the catalyst is taken of the aluminum phosphate.

Usage
Methanediol – aqueous solution of formaldehyde causing the denaturation of proteins. This quality allows to use this material as a tanning agent in the manufacture of leather things. He also found wide application in the manufacture of artificial fiber, film. Because of the strong tanning properties formaldehyde is known as an antiseptic. It is commonly used in medicine. On its basis, produce such antiseptic preparations as “Formagel” and “balusters: stone”. For quite a long time formic aldehyde is used in biology for the storage of biomaterials, e.g., organs or corpses of animals.
Currently obtaining formic aldehyde profitable woodworking and, of course, the furniture industry. It is used for the production of melamineformaldehyde resins. They are used in the manufacture of particleboard, plywood and other construction materials.
When storing formic aldehyde note that at temperatures below 10 degrees, the solution becomes cloudy and a white precipitate appears. Formaldehyde also reacts with oxygen. As a result, because of the degenerate branching additional initiators of the chain.
Toxic properties
So, formaldehyde is produced by oxidation of methanol and methane. Sure, he has a fairly high degree of toxicity. Formic aldehyde primarily affects genetic material. From this substance will suffer and reproductive organs. But, of course, often marked by the defeat of the respiratory tract, skin and eyes.

Dangerous formaldehyde because it has a negative effect on the Central nervous system.
Formic aldehyde is not just toxic - 60-90 ml (depending on body weight) ingested, will cause death. Symptoms of poisoning the following:
- General fatigue;
- Pallor;
- Unconsciousness;
- CNS depression;
- Difficulty breathing;
- Headache;
- Cramps, especially at night.
The Impact of formic aldehyde on the human body

The Use of formic aldehyde in the production often causes varying degrees of poisoning among employees. When severe inhalation injury, i.e. by inhalation substances, occur conjunctivitis and the acute form of bronchitis, which sometimes leads to edema of the lung. Symptomatology during prolonged exposure to formaldehyde will only increase. After a short time there are signs of damage and CNS depression. It will be expressed in constant dizziness, there is a sense of fear, gait becomes shaky, and at night there may be even convulsions.
If the poisoning occurred through the mouth, the first symptom is a burn of the mucous membrane of the digestive tract. It will manifest as pain, burning sensation in throat and duringof the esophagus. The person feels a strong retching, mass, rejects the stomach will contain blood. In severe cases, edema of the larynx and there is a reflex stop breathing.
In chronic poisoning with low concentrations in humans observed strong weight loss, mental excitement, visual disturbances, insomnia and constant headaches.
The Poisoning of formaldehyde
Formic aldehyde is most often found in the air. Employees of enterprises engaged in the work connected with the manufacture of synthetic resins, in the presence of constant and prolonged contact with vapors of formaldehyde may poisoning. Often already in the first days does a person get visible dermatitis on the face, hands. The presence of formaldehyde in the body can be seen on the deterioration of the nails - they soften and become brittle.
Eczema and dermatitis can wear allergic in nature. After a man suffered a poisoning, it is formed by formic aldehyde to the strong sensitivity. There is evidence that formaldehyde adversely affects the functioning of the reproductive system of the female body.
The Use of formaldehyde in cosmetic products

The Content of formaldehyde is allowed as a preservative in cosmetic products, but its concentration should not exceed 0.1%. Formic aldehyde can be present in toothpaste, body creams, face and hands.
Also in pharmacology, in some means to prevent sweating, may be formaldehyde. Its allowable concentration is 0.5%. This substance is a good disinfectant, even in such small quantities he is able to destroy microorganisms. It is strictly forbidden to put on the face of any ointment, if it is 5% formaldehyde. It is fraught with the appearance of dermatitis and allergic reactions. Also formic aldehyde is not used for preservation of cosmetic products if they are sold as sprays and aerosols.
All products on the label must contain information regarding the content of this hazardous substance, even if the amount of 0.05%. In fact to this day not clarified the effect of formaldehyde solution on the skin, but it is known that in animals, it becomes red and scaly.
Carcinogenicity formic aldehyde
It is Known that formaldehyde interacts with the selenium with the participation of highly concentrated sulfuric acid. The result is sulfoethyl-4-tetradecylbenzene. This substance with the complicated name after treatment with hydroxide of barium becomes the perfect cleaner. It would seem, what's the danger? But formaldehyde is included in the list of substances with carcinogenic qualities. Although the degree of risk is not yet established, it is impossible not to take into account the fact that formic aldehyde is deadly for animals. According to official data of many scientific centers of the world, the relationship between the use of formaldehyde in the manufacture of paints, resins, textiles, plastics, and the emergence of cancer tumors in humans proven. A particularly high risk of developing cancer of the nasopharynx.
Article in other languages:
AR: https://www.tostpost.com/ar/education/4962-formic-aldehyde-obtaining-formic-aldehyde.html
DE: https://www.tostpost.com/de/bildung/8847-ameisens-ure-aldehyd-erste-aldehyd-murav-inogo.html
HI: https://www.tostpost.com/hi/education/4967-formic-aldehyde-obtaining-formic-aldehyde.html
JA: https://www.tostpost.com/ja/education/4963-formic-aldehyde-obtaining-formic-aldehyde.html
KK: https://www.tostpost.com/kk/b-l-m/8850-murav-inyy-al-degid-alu-murav-inogo-al-degid.html
PL: https://www.tostpost.com/pl/edukacja/8849-mr-wkowy-aldehyd-otrzymywanie-aldehydu-mr-wkowego.html
PT: https://www.tostpost.com/pt/educa-o/8845-murav-inyy-alde-do-receber-murav-inogo-um-alde-do.html
TR: https://www.tostpost.com/tr/e-itim/8854-kar-nca-aldehit-alma-murav-inogo-aldehit.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
An archaeologist is... the Profession of the archaeologist. Archaeologists
Mention of archaeology began in Ancient Greece. For example, Plato under this term is understood the study of antiquity and in the Renaissance meant the study of the history of Greece and Ancient Rome. In foreign science, the term...
Forms of carrying out of class hours in school
the Homeroom is an important form of the educational work in the classroom. This form is characterized by its flexibility. It can actively affect the children, to try to develop their positive qualities.Goals of the class hourchoo...
What is oblique seven feet, and if it is shoulders?
since time immemorial to characterize heroes or just a big man, said: “broad shoulders.”. What is it – seven feet? If accurate, this definition of the width of the chest or artistic hyperbole? Let's face it. Afte...
Possible evidence for the existence of God?
In our secular age more and more people talking about the fact that faith need proof of God's existence. For the person deeply believing there is a God, and it is not necessary to prove neither this man nor God. For the atheist th...
Rhymes with "sadness" for the authors of the poems
Rhymes with “Sad” may be required in different works. Why every author should have in his notebook of ideas that need to use.Rhymes with “sad”In lines of poetry should be a consonance. Rhymes with “sa...
Healthy blood vessels: a life without cardiovascular disease
the Blood in our body serves as a connecting element providing activity of each cell, each organ. Its movement in the body is called circulation, by which oxygen, hormones and nutrients act in all tissues and organs, and from ther...






















Comments (0)
This article has no comment, be the first!