Obtaining a sulfide, its properties, application
In this article, we consider obtaining of hydrogen sulfide from sulfur. Take a closer look at the physical and chemical properties of the substance.

Structure
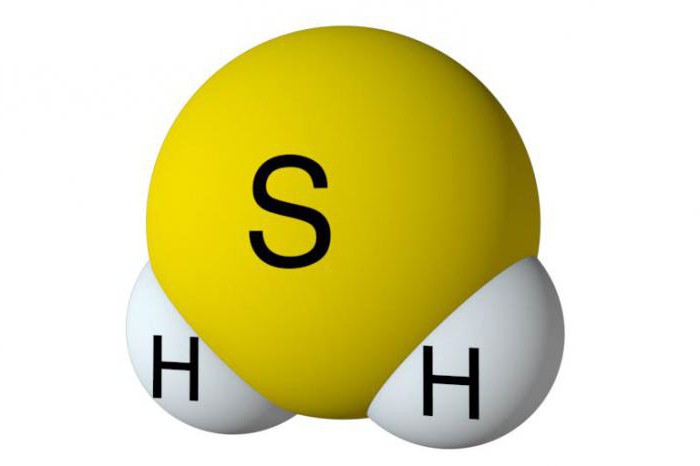
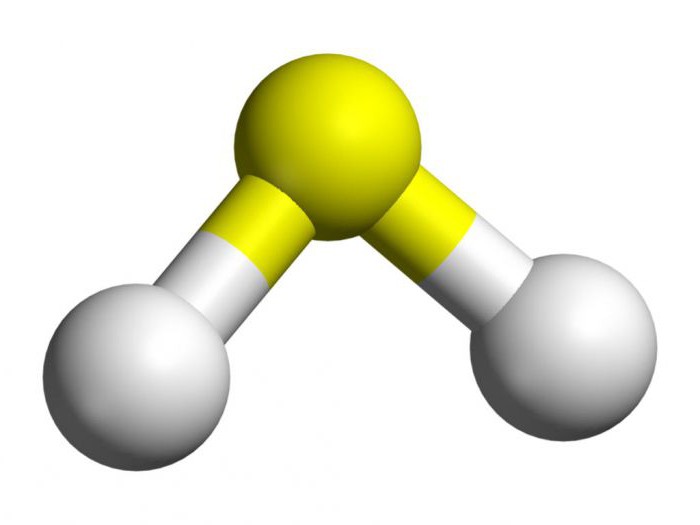
In order to perform the basic obtaining of hydrogen sulfide, it is necessary to find out the specifics of its structure. In the composition of this substance contains one atom of sulfur and two hydrogens. They are nonmetals, so between the elements are formed by covalent polar. Hydrogen sulfide in the corner of the building. Between sulphur and hydrogen form an angle of 92 degrees, which is slightly less than in water.
Physical properties
Hydrogen sulfide Odor, reminiscent of rotten eggs, is familiar to all. Under normal conditions this substance is in gaseous state. It has no color, poorly soluble in water, poisonous. On average at 20 degrees Celsius in water will dissolve 2.4 volume of hydrogen sulfide. Hydrogen sulfide from water revealed slight acidic properties, dissociation, substance flows step. Toxic hydrogen sulfide is dangerous even in small doses. The contents in the air for about 0.1 percent of hydrogen sulfide causes paralysis of the respiratory center with loss of consciousness. For example, the legendary naturalist Pliny the Elder died in 79 BC, it is the hydrogen sulfide which was formed during the eruption of Vesuvius.
The Cause of the toxic action of hydrogen sulphide in its chemical interaction with hemoglobin. The iron contained in this protein forms a sulfide with hydrogen sulfide.
The Maximum allowable concentration in the air of hydrogen sulfide is considered to be 0.01 mg/L. as an antidote is used, the inhalation of pure oxygen or of air, which is composed of minor amount of chlorine.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
Working with hydrogen sulfide involves compliance with certain safety regulations. All the experiments pertaining to this gaseous substances are carried out in sealed equipment and fume hoods.

Ways of obtaining hydrogen sulfide
What is the getting of hydrogen sulfide in the lab? The most common approach is interaction of hydrogen with sulfur. This chemical reaction belongs to the Union, is carried out in a fume hood.
In addition, the receipt of hydrogen sulfide and possibly in the exchange between solid iron sulfide (2) and a solution of sulfuric or hydrochloric acid. To obtain such a result, in the tube, it is sufficient to take a few pieces of sulfide, not exceeding the size of a pea. Further, in the tube (half of it) was added to a solution of the acid, close the vapor tube. The device is placed under the hood, the tube is heated. Chemical interaction is accompanied by release of gas bubbles. Such receipt of sulfide allows you to create a quantity of the substance sufficient for consideration of its chemical properties.
What are the ways? In the laboratory allowed the obtaining of hydrogen sulfide by reacting metallic iron (under the hood) with crystalline sulphur, with the subsequent interaction of sulfide with sulfuric acid.
Chemical properties
Hydrogen Sulfide reacts with oxygen of air, it burns with a bluish color. In the case of a complete combustion reaction products are sulfur oxide (4) and water. Given that the furnace gas is acidic oxide that in solution it forms a weak sulfurous acid, coloring blue litmus paper red.
In case of insufficient amount of hydrogen sulfide formed a crystalline sulfur. This process is considered an industrial method of producing clean hydrogen sulfide from sulfur.
The chemical is identified and excellent resilience. They appear, for example, when interacting with salts, Halogens. In order to carry out in laboratory conditions similar to the reaction in the tube with chlorine and bromine is poured a solution of hydrogen sulfide, see the discoloration. As reaction product observed the formation of crystalline sulfur.
When the chemical reaction of hydrogen sulfide with water causes the formation of hydronium cation Н3О+.
The hydrogen Sulfide is able to form two types of compounds: sulfides (average salt) and hydrosulphide (sour salt).
Alkaline, and alkaline earth metal sulphides are colourless compounds. The heavy metals (copper, Nickel, lead) they are black. The manganese sulfide has a pink color. A salt not soluble in water.
Qualitative reaction to the sulphides consider the interaction with the copper sulfate solution (2). The product of such interaction is the deposition of black precipitate of copper sulfide (2).
Conclusion
The nature of this substance is in the mineral springs, volcanic gases. This compound is a product of the putrefaction of animal and vegetable organisms, it is distinguished by the characteristic odor of hydrogen sulfide. Natural sulphides found in the rare metals, metallurgy of them receive the relevant elements. It is important to remember that hydrogen sulfide is a strong poison gas.
Article in other languages:
AR: https://www.tostpost.com/ar/education/17470-obtaining-a-sulfide-its-properties-application.html
HI: https://www.tostpost.com/hi/education/19114-obtaining-a-sulfide-its-properties-application.html
JA: https://www.tostpost.com/ja/education/17134-obtaining-a-sulfide-its-properties-application.html
KK: https://www.tostpost.com/kk/b-l-m/33958-alu-k-k-rtt-suteg-ony-asietter-oldanyluy.html
TR: https://www.tostpost.com/tr/e-itim/30374-alma-hidrojen-s-lf-r-zellikleri-uygulama.html
ZH: https://www.tostpost.com/zh/education/2733-obtaining-a-sulfide-its-properties-application.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
Why in the reformation there were different directions? The reformation and its major trends
In the middle Ages the power of the Church became a dominant force not only in spiritual but also in the political sphere of society. Preaching abstinence, humility, Church Ministers profited from the indulgences, tithes, levies f...
The meaning of the word "tribe". Causes of tribes
Life on Earth began very long ago, namely about 3.7 billion years ago. The evolution continues today. The person is not standing still and is constantly evolving. Today we live in a modern society, and in ancient times people live...
The interpretation, definition and meaning of the word "developed"
Truly a wonderful Russian language! And for all his wealth, there are words that are very similar, but the meaning, stress that their origin is different. So there are difficulties in the interpretation of certain expressions, and...
The Nazarbayev University in Astana
the Nazarbayev University in Astana was created only six years ago. During this short period the University has established itself as an elite institution, combining the original traditions with modern training and the best educat...
The food chain in the forest. General concepts and examples
Most organisms feed on organic food, this is the specificity of their activity on our planet. Among this food, and plants and meat of other animals, their products work and dead matter, ready for decomposition. The process of nutr...
What formal languages can be attributed? Examples of usage
What is the formal language and how it differs from the natural? How it was formed? What formal languages can be attributed? And what is its designation?Characteristics of formal languagesthe So called group of artificial language...




















Comments (0)
This article has no comment, be the first!