Bertoletova salt
What is potassium chlorate?
The Potassium salt of chloric acid (one of the four oxygen-containing acids formed by the chlorine: hypochlorous — HClO, chloride — HClO2, chloric — HClO3, and perchloric -- HClO4) is called potassium chlorate, its formula — KClO3. This salt in appearance is a crystal (colorless), which is poorly soluble in water (at 20 ºC in 100 cm3 of water dissolves only 7.3 g of salt), but with increasing temperature the solubility increases. Another famous name — bertoletova salt. The molecular mass of the substance is 122,55 atomic mass units, a density of 2.32 g/cm3. Salt melts at 356 ºC, decomposes at about 400 ºC.
The Discovery of potassium chlorate
For the First time (in 1786) potassium chlorate was awarded to the French chemist Claude Bertolli. He passed chlorine through hot concentrated solution of potassium hydroxide. The equation of the reaction that was received salt, as follows: 3Cl2 + 6KOH → 5KCl + KClO3 + 3H2O. The result of this reaction potassium chlorate precipitates as a white precipitate. As it is poorly soluble in cold water, it is easily separated from the remaining salts during cooling of the solution. Since its opening bertoletova salt is the most common and useful product of all chlorates. Currently KClO3 produced on a commercial scale.
Chemical properties
Potassium chlorate salt — strong oxidiser. At its interaction with concentrated hydrochloric acid (HCl) produces free chlorine. This process is described by the equation of chemical reaction: 6HCl + KClO3 → 3Cl↑ + KCl + 3 H2O. Like all chlorates, this substance is highly poisonous. The molten KClO3 vigorously supports combustion. In mixture with easily oxidizable substances (reductants), such as sulphur, phosphorus, sugar and other organic substances potassium chlorate explodes on impact or friction. Sensitivity to these stimuli is enhanced in the presence of ammonium salts and bromato. With careful (heating to 60 ºC) oxidation of potassium chlorate with oxalic acid you get chlorine dioxide, the process proceeds according to the reaction equation: 2KClO3 + H2C2O4 → K2CO3 + CO2 + H2O + 2ClO2. The oxide of chlorine is used in bleaching and sterilization of various materials (paper pulp, flour, etc.), and can also be used to obespylivanija wastewater chemical plants.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
The Use of potassium chlorate
Of all the chlorates potassium chlorate salt finds the widest application. It is used in the manufacture of dyes, matches (make combustible match head, raw material is the hydrated potassium chlorate on the other 6-18-24-84), fireworks, disinfectants, chlorine dioxide. Because of the high risk of compounds with chlorate of potassium, they are practically not used in the production of explosives for industrial and military purposes. Very rarely potassium chlorate is used as initiating explosives. Sometimes used in pyrotechnics, the result is zitopenia compositions. Before salt used in medicine: weak solutions of this substance (KClO3) a time used as an antiseptic for external gargling. Salt in the early 20th century used to produce oxygen in laboratory conditions, but because of the danger of experiments and they were terminated.
Making potassium chlorate
One of the following methods: chlorination of potassium hydroxide, as a result of exchange reaction of chlorates with other salts, electrochemical oxidation of aqueous solutions of chlorides of metals — can be obtained bertoletova salt. Obtaining it on an industrial scale often carried out by the reaction of disproportionation of hypochlorites (salts of hypochlorous acid). Technologically, the process execute in different ways. Often it is based on the reaction between calcium chlorate and potassium chloride: Ca(ClO3)2 + 2KCl → 2KClO3 + CaCl2. Then, the resulting bertoletova salt from the mother liquor is allocated by the method of crystallization. Also potassium chlorate get by the modified method of Bertolli in the electrolysis of chloride of potassium formed during the electrolysis, the chlorine interacts with potassium hydroxide, the resulting potassium hypochlorite KClO disproportionate then potassium chlorate KClO3 and the source of potassium chloride KCl.
The Decomposition of potassium chlorate
At a temperature of about 400 ºFrom the decomposition of potassium chlorate. The result is oxygen and potassium perchlorate: 4KClO3 → KCl + 3KClO4. The next stage of decomposition occurs at a temperature of from 550 to 620 ºC: KClO4 → 2O2↑ + KCl. The catalysts (they can be copper oxide CuO, iron (III) oxide Fe2O3 oxide or manganese (IV) MnO2) decomposition proceeds at a lower temperature (from 150 to 300 ºC) and in one phase: 2KClO3 → 2KCl + 3O2.
Security Measures
Potassium chlorate salt is volatile explosive chemical that can explode when mixing, storing (for example, a reducing agent on the same shelf in the laboratory or in one warehouse), grinding or other operations. The explosion may occur injury or even be followed by death. Therefore, upon receipt, use, storage or transport of potassium chlorate must comply with the requirements of the Federal law 116. The objects for which organized these processes, refer to hazardous industrial facilities.
Article in other languages:
AR: https://www.tostpost.com/ar/cars/7586-what-is-the-exam-in-the-traffic-police.html
BE: https://www.tostpost.com/be/a-tamab-l/13550-shto-zh-uya-lyae-saboy-ekzamen-u-d-bdr.html
DE: https://www.tostpost.com/de/autos/13553-was-ist-eine-pr-fung-in-der-verkehrspolizei.html
ES: https://www.tostpost.com/es/coches/13560-que-es-un-examen-en-el-gbdd.html
HI: https://www.tostpost.com/hi/cars/7592-what-is-the-exam-in-the-traffic-police.html
JA: https://www.tostpost.com/ja/cars/7588-what-is-the-exam-in-the-traffic-police.html
KK: https://www.tostpost.com/kk/avtomobil-der/13553-b-l-b-ld-red-emtihan-gibdd.html
PL: https://www.tostpost.com/pl/samochody/13540-co-to-jest-egzamin-do-policji.html
PT: https://www.tostpost.com/pt/carros/13534-o-que-um-exame-de-pol-cia-de-tr-nsito.html
TR: https://www.tostpost.com/tr/arabalar/13556-nedir-bu-s-nav-traf-k-polisi.html
UK: https://www.tostpost.com/uk/avtomob-l/13550-scho-zh-yavlya-soboyu-spit-v-gibdd.html
ZH: https://www.tostpost.com/zh/cars/8252-what-is-the-exam-in-the-traffic-police.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
Why Lenin was not buried immediately after death? The views of historians
Now 90 years old, died changed the course of Russian history in the last century, the man some people praise God, others – curse. It – V. I. Lenin. But still no fall silent disputes about why Lenin buried?the Fate of t...
Drift is the condition of the ship, car, sailboat. The meaning and interpretation of
To describe the progressively uncontrollable movements use the concept of “drift”. This word is often used by sailors, electricians, race car drivers, doctors. In English the drift means “flow”, “for&...
A Fibonacci level in currency trading: common mistakes and best practices for building
Almost every trader with even the most minimal experience in the trade, for once in my practice have tried to use this very useful tool. Usual Fibonacci used to determine the start points of a possible correction and prediction of...
As indicated by distance in physics? Interesting examples
the Theme is dedicated to those students who have physics first year. Here we talk not only about how distance is denoted by in physics, but also about other interesting things. Let this subject be interesting for all sections and...
The major cultural achievements of Ancient Egypt
we Know that the cultural achievements of Ancient Egypt and ancient tsivilizatsii became a base through which in subsequent centuries came the development of both the European and world scientific and technical progress. Many revo...
day of November 1472 in Moscow was a lot of commotion – came to the capital the Tsar's bride Sophia Palaeologus. A few days later at the assumption Cathedral was held her wedding with Ivan III, who was widowed five years ear...


















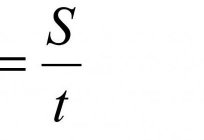


Comments (0)
This article has no comment, be the first!